Summary
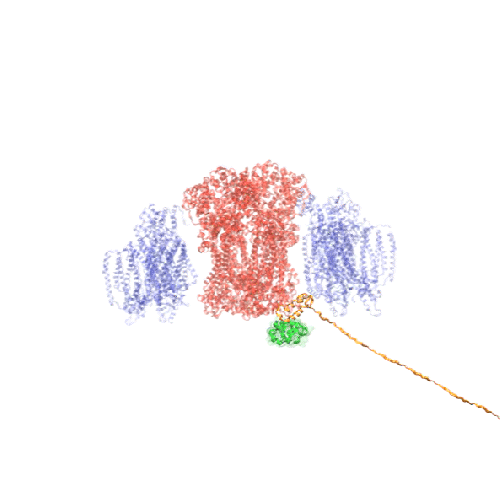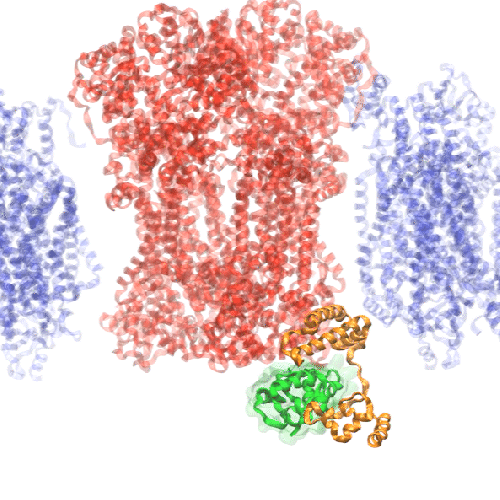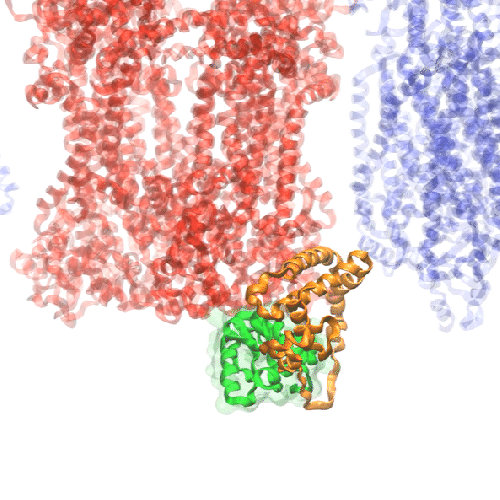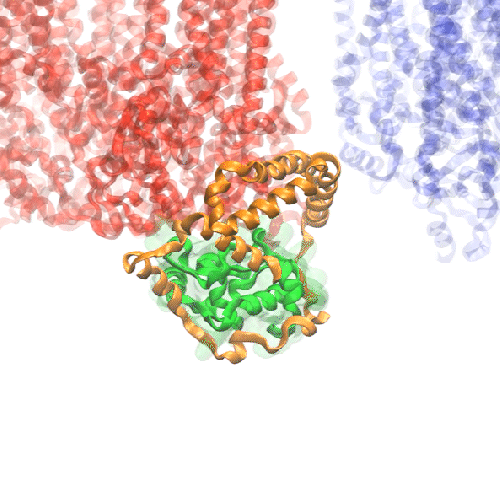
The Network of Optimal Dynamic Energy Signatures (NODES) program aims to develop a groundbreaking deep learning tool, informed by the principles of biophysics, that can analyze vast numbers of protein sequences and predict their biological functions by identifying telltale patterns of protein movement.
With this program, DARPA seeks to characterize the function of potential biological threats within one hour, expanding threat assessment accuracy and speed exponentially. Speed is crucial for developing effective medical countermeasures for warfighters and for staying ahead of advancements in biotechnology that could lead to new and emerging biothreats.
Additionally, NODES will provide a secure system for classifying and monitoring biological threats, which is vital for intelligence agencies to enhance bio-surveillance.
Building protein understanding in motion
FAQs
Last updated: Aug. 20, 2025
General information
Q: Has the Program Solicitation been published? Where is it posted?
A: Yes, it was published on July 31 2025, and can be found on SAM.gov.
Q: Will the Proposers Day slides be posted online?
A: Yes, Dr. Singharoy’s program overview provided during the Proposers Day has been made available on the NODES program page.
Q: Is Dr. Singharoy available for a call or meeting to discuss how our technology may align with the program?
A: In the interest of fairness to all interest parties, as Dr. Singharoy will not have the availability in his schedule to honor all requests, we will not be scheduling and program-related calls or meetings. The best way to receive feedback on an approach is through the submission of a proposal abstract prior to the deadline specified in the Program Solicitation (PS). The PS describes the program, including metrics, in detail. Specific questions may be submitted by email to NODES@darpa.mil. Proposers should be aware that submitted questions and answers may be published on an FAQ page, with revisions to remove proprietary information.
Q: Is teaming required?
A: While teaming is not required, it is strongly encouraged to provide the expertise and capabilities needed to achieve the NODES program goals. Proposing teams should have a plan in place for managing team interactions and future technology transitions.
Q: Could you clarify Teaming in general. Can teams be made up of Prime and Subcontractors with the Prime organization addressing all three FAs and the subcontractors supporting the effort. Or Is the Prime and Subcontractors team expected that each member of the team will address all three FAs?
A: The team composition is up to the proposers. All teams before or after the formation of superteams will work together to satisfy all three FAs in whatever way they see fit. The proposers should decide how to best have the FAs addressed.
Q: Can individuals be part of multiple proposals under this solicitation, or are there any restrictions regarding team member overlap across different submissions?
A: Yes. Teams can be subcontractors on multiple efforts. However, if chosen for multiple awards, a clear path will be established to ensure no conflicts are present between the efforts. Proposers who are subcontractors on multiple teams should be cognizant of the distribution of the level of effort across multiple awards and will be required to ensure that DARPA is only charged once for any potential duplicate tasking.
Q: Are individuals without an affiliation to an organization eligible to submit an abstract?
A: No. Refer to Section 6.1 of DARPA-PS-25-30, where it is described what an entity needs to be eligible for an award.
Q: Is it possible for a team that doesn't include a typical Prime Contractor or University to win, such as a consortium of small entities? Do you reserve any slots for unconventional teams like this?
A: Yes - submissions will be evaluated using the criteria listed in Section 4.3 of the Program Solicitation. Performer slots are not reserve for any specific organization type(s).
Q: Should the proposals have one PI or can they have multiple PIs?
A: It is expected that the proposer will propose the correct number of personnel required to support their technical approach.
Q: Can foreign entities/organizations participate in the program?
A: No. As stated in Section 3.1.2 of DARPA-PS-25-30: “Non-U.S. organizations and/or individuals are NOT eligible to submit an abstract/proposal to this solicitation. Non-US individuals employed by US organizations and working in the US are allowed to participate on the Performer teams.”
Contracting and cost
Q: How much funding is available for the NODES program? What is the expected size of an award? Approximately how many projects is DARPA planning to fund?
A: Total costs must not exceed $1.7M of Government funding.
Q: Do I need institutional sign off for my estimated budget?
A: The abstract template requests an “estimated cost” and provides guidance as to what that entails. As to whether you need institutional sign off on that estimate is a determination for your organization to make.
Q: Can DARPA partially fund a proposal?
A: Yes, DARPA reserves the right to fully or partially fund a proposal.
Q: Will funding for performers to run their own experimental assays to validate and train their models be an allowable cost under the NODES solicitation?
A: Yes.
Q: Should the estimated cost include the use of T&E government partner’s computing facility?
A: Use of the T&E government partner facility is paid by the U.S. government by those who wish to use it and not needed to be added in the proposer’s cost. In that case, proposers must state how much computational power is needed for their efforts.
Q: What types of award mechanisms will be executed under the NODES program?
A: Other Transaction for Prototype.
Q: Is a budget request to purchase additional compute power for the Co-PIs lab allowed? Is a budget request to pay for "fee for use" access to centralized research computing power at the prime university allowed?
A: The offeror’s budget should be well-justified and reflect the proposed technical approach.
Q: Does DARPA expect proposers to use the government provided HPC resources - unless they can justify a compelling reason why they cannot?
A: It’s acceptable for proposers to use their own computational resources. However, the final product, as well as capability demonstrations, will have to run and be executable on government HPC. Program Structure
Program structure
Q: Is experimental data generation supported (e.g., links between structure/dynamics and protein function) or is the focus purely computational?
A: The original focus of NODES was purely computational; we do have government partners for performing experiments and validation. However, proposed experiments that are feasible within the budget and timeline proposed by BTO, and lead to outcomes are generalizable beyond the experimental systems will be deemed within scope.
Q: Will the National Labs or other government partners be supplying data to performers to assist in training their model and validating simulations during Phase I (outside of the capability demos)?
A: The government partners will not be supplying training data for training models, only sequences for Capability Demonstrations at 6 and 9 months.
Q: Are government computing resources free to use and on demand?
A: Yes, but within limited GPU resources, as mentioned in the PS. This is a key part of the research plan that should be rationalized in the abstract.
Q: Are performers required to provide software licenses for running commercial software on government resources?
A: Yes.
Q: What IP rights, if any, does DARPA anticipate for work products from NODES? What about for underlying IP created before the program but needed for program work? Could you please clarify the protection for companies developing proprietary models and how they will be deployed by the USG? Will it be possible to negotiate terms to protect commercialization rights of software first generated during the program?
A: See Sections 6.3 and 6.4 of DARPA-PS-25-30. Negotiation of terms is possible – please note that should the negotiating parties not be able to come to terms after a team has been notified of their selection, DARPA is not required to make an award.
Q: What would be the differences between classified and CUI forms of the expected app?
A: Once the code can start to model binding, the software will become CUI. A final version will be at the collateral level once it has been used to model classified proteins. Abstracts and Proposals
Q: Could you expand/define “function”? To what level of specificity are you referring?
A: Protein function is a concept that can have different interpretations in different biological contexts. Generally, it describes biochemical, cellular and phenotypic aspects of the molecular events that involve the protein, including how the protein interacts with the environment (such as with small compounds or pathogens). From the various classification schemes developed to standardize descriptions of protein function, we chose the “Molecular Function” and “Biological Process” categories from Gene Ontology (GO). Each category in GO is a hierarchical set of terms and relationships among them that capture functional information; such a system facilitates computation, and its outputs can be interpreted by humans. GO's consistency across species and its widespread adoption make it suitable for large-scale computational studies. In NODES, given a new protein sequence + structure + movement, the task of a protein function prediction method is to provide a set of terms in GO along with the confidence scores associated with each term.
Abstracts and proposals
Q: Is submitting an abstract required?
A: Yes. Proposers must submit an abstract in response to this solicitation to be considered for participation in the NODES program. Proposers will not be invited to submit an OPP, provide an oral presentation, or be included in any further progression of the program without participating in the abstract phase of the solicitation.
Q: Should I submit via email, DARPA’s BAA Portal, or Grants.gov?
A: Proposal Abstracts must be submitted via DARPA’s BAA Portal.
Q: Do all key personnel have to be identified prior to the submission of an abstract?
A: DARPA understands that final concepts and team make-up may change from abstract phase to oral presentation as the technical approach is solidified, however, please note that “Technical Ability” (as defined in Section 4.3 of the PS) is one of the evaluation criteria for proposal abstract submissions.
Q: Could experimental assays to validate and train the models be part of the proposed solution/abstract and be subcontracted to the team that is being formed?
A: Yes, however, the team needs to be able to demonstrate they can complete all milestones and achieve all computational metrics requested within budget and in time.
Q: How focused are proposals expected to be? Is it of interest if a team proposes to focus specifically on predicting the likelihood of pathogenicity or prediction of specific functions (e.g., DNA-binding)? Or is the tool expected to be as general as possible?
A: Please refer to Section 4.1 of DARPA-PS-25-30.
Technical questions
Q: Are RNA, protein modifications, and non-protein biomolecules included?
A: The focus of NODES is discoveries of novel protein functions. In the context of this broader goal, if a method allows for extension to other biomolecules or small molecules, it is acceptable. But our capacity demonstrations in Year 1 will only test protein properties.
Q: Are countermeasure discovery or intervention pipelines part of the envisioned scope?
A: Not in year 1. MCM discovery can be mentioned by proposers in the section of the abstract devoted to Phase II. Nonetheless, this page will not be evaluated in the current solicitation.
Q: In the NODES solicitation, it mentions that it is necessary to have exedynamic weights for each protein conformation (rather than arbitrary scores from a model). Would thermodynamic weights derived from (accelerated) molecular dynamics using a neural network potential (as opposed to a traditional forcefield) qualify? Is the intention to just ensure that the weights reflect the experimental data as best as possible?
A: There is no restriction on the choice of methods if it is ultimately directed to determining function. Computed weights will be compared with experimental results.
Q: According to the materials presented the goal of Phase I of the NODES program is to "reproduce the functions of 15-20 known multifunctional proteins" via protein dynamics. Is the design and prediction of protein-protein interactions still a fit for the NODES program?
A: Yes, protein-protein interactions are within the realm of the program if they can be associated with both protein sequence as well as function.
Q: Can a proposal focus on a single family of proteins (e.g., GPCRs). Or if not a single family, a combination of proteins, such as GPCRs, transporters, and ion channels?
A: A specific target protein-oriented work is non-conforming to Year 1. But a proposal can be deemed conforming if the approach developed based on a single family is generalizable enough, so it can handle the CD1 and CD2 tests with desired accuracy.
Q: What level of function prediction is the program hoping to achieve?
A: Gene Ontology for now. More detailed queries on biological threat will be considered in Phase 2.
Q: What are the expected data volumes (100,000 structure-function links? more, less), validation expectations?
A: There is no restriction on data volume. If by using 100,000 structure-function links, the proposer’s algorithm can achieve 60% accuracy in CD1 and 70% accuracy in CD2, then it is acceptable.
Q: What range of molecular weights (and whether monomers vs. complexes) should we assume for the envisioned protein targets?
A: Proposers are to select methods they find appropriate.
Q: Can you share the expected origin of the protein sequences (organismal source) and anticipated biosafety level?
A: The methods should be agnostic to organism, as the sequences provided may come from human or microbial organisms and may range several causative agents of infectious disease.
Q: Would this opportunity fulfill data generation for model development? For example, throughput DNA-encoded library screening for protein-small molecule binding or SPOC/HuProt technology for protein-protein binding?
A: As long as it can be done with the time and budget limits, but most importantly, the data is employed to learn sequence-to-function relationships.
Q: Are the “molecular dynamics trajectories” listed in several deliverables analogous to “model outputs”?
A: Yes, “molecular dynamics trajectories” as “model outputs” is a correct assessment.
Q: Capability Demonstration 1 is stated to include assessments of Functional Areas 2 (binding) and 3 (allostery). Could you clarify the provided inputs and desired outputs for the capabilities to be assessed? Will the identity of binding partner or allosteric effector of the protein be provided? If not, are performers expected to predict the identity of the binding partner or allosteric partner?
A: The identity of binding partners or other proteins will be provided in Capability Demonstration 1.
Q: Could you provide a rough estimate on the number of proteins that we will be provided to build the initial models?
A: Teams will not be provided with proteins to build models. Models are expected to be generic. At Capability Demonstration 1, teams will receive a set of proteins to verify and validate that their models work.
Q: As stated in Capability Demonstration 1, performers are expected to predict the binding constants, interfaces, and conformations. Does this refer to the protein in isolation (e.g., predict which residues are interacting with the ligand), or of the protein-ligand complex (e.g., predict the structure of the complex)?
A: Apo states will be tested in Phase I by performers.
Q: The milestones state that performers are expected to deliver a “% partial completion of protein movement libraries” in the form of “molecular dynamics trajectories and accompanying files.” What does this % refer to?
A: It refers to a protein database proposed by the submitting organization.
Q: Are performers expected to deliver molecular dynamics trajectories for proteins in the target set, or should this language be interpreted as broadly referring to libraries of dynamics signatures to be used for function prediction?
A: The latter.
Q: Methods are limited to <=300 GPU hours per protein at inference time. Are methods that are more GPU-efficient more desirable, or are further improvements in efficiency and throughput not of importance? What is the amount of compute in GPU hours we can assume will be provided by government partners?
A: Further improvements are of interest. Applicants are to include in their proposals how many GPU hours they will need and are requesting of government partners.
Q: Could you please clarify the following for the protein targets in CD1 and CD2: Sequence Length (Size): What is the typical or expected size range of the proteins (e.g., number of amino acids or molecular weight)?
A: The size will range from 100 – 1000 amino acids.
Q: For the protein targets in CD1 and CD2, should we expect these proteins to be monomeric, homo-oligomeric, or hetero-oligomeric?
A: Yes, all three should be expected.
Q: Will the sequences in CD1 and CD2 include other categories beyond membrane proteins (e.g., soluble enzymes, multi-domain scaffolds, intrinsically disordered proteins)?
A: Yes.
Q: Is MD simulation a necessary component in the proposal?
A: No, in the sense that Molecular dynamics extends beyond classical MD simulations.
Q: Can you elaborate what you mean exactly by “thermodynamics, energy, and force field-based weights”?
A: These are standard parameters in molecular dynamics.
Q: Could you clarify how to interpret 60% or 70% accuracy for metrics in tests that are not classification (month 6, month 9 deliverable/metric) such as Root Mean Square Deviation, thermodynamic weights, pLDDT scores, or binding constants?
A: As achieving 60%-70% of the values currently found in the published literature or provided experimentally
to performers after the Capability Demo.
Q: Utility of single-molecule dynamics (FA1): is the hypothesis that identifying intrinsically flexible domains/regions/surfaces (or intrinsically static…?) will be predictive of binding pockets and/or PPI interfaces?
A: Single-Molecule dynamics have many goals, for example, to find different folding states that have functions beyond what is stated above. Ultimately, it is predictive of mechanisms and hence functions.