In Vitro Platform Technology to Rapidly Assess Medical Countermeasures
The Microphysiological Systems (MPS) program supports military readiness by enabling timely evaluation of the safety and efficacy of novel medical countermeasures against a wide range of natural and man-made health threats, including emerging infectious disease and chemical or biological attack.
Testing these types of countermeasures is particularly challenging using current methods because it is often both unethical and impractical to evaluate countermeasures using human clinical trials. Instead, the U.S. Food and Drug Administration (FDA) must base its determination of efficacy and toxicity on data from animal studies, despite the fact that animal models have limited relevance to humans and poorly predict effects in humans.
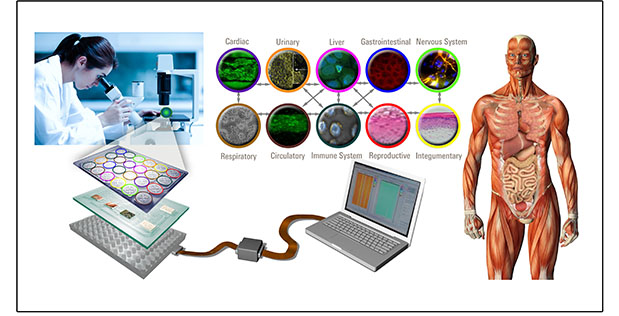
To overcome this challenge, the MPS program is developing in vitro platform technology to rapidly assess medical countermeasures in a way that is relevant to human health using interlinked “organoid” systems that incorporate engineered human tissue and microfluidics technology into microchips that mimic the functions of human physiological systems.
MPS performer teams are working specifically to develop a reconfigurable platform that permits simultaneous study of ten or more interlinked in vitro physiological systems, arranged in any sequence, with the ability to sustain tissue for up to four weeks to evaluate effects of countermeasures over time.
The teams must demonstrate that the engineered tissues function together to accurately reproduce both the human physiological systems they are intended to mimic and the biological crosstalk that occurs among systems. To validate the platform’s predictive ability, teams are testing compounds with known effects in humans. Related research is applying infectious agents to the platform to understand if the physiological effects of health threats can be modeled to facilitate development of new countermeasures.
DARPA involved the FDA from the beginning of the MPS program to help ensure that regulatory challenges of reviewing drug safety and efficacy are considered during development of the MPS platform. DARPA is also coordinating efforts with the National Institutes of Health, which is conducting separate but parallel research.
If the MPS program is successful, the resulting platform should decrease the time for development and increase the number and quality of medical countermeasures to bio-threat agents that move through the FDA pipeline and into clinical care.
This program is now complete.
This content is available for reference purposes. This page is no longer maintained.
