Prototype’s novel approach uses magnetic nano-sized beads to filter deadly microbes and toxins from the bloodstream
Sep 15, 2014
Sepsis—a life-threatening over-reaction by the immune system to infection—afflicts 18 million people a year worldwide and kills between 30 and 50 percent of them. Sepsis poses a significant threat to warfighters who suffer combat injuries that predispose them to infection. Antibiotics can kill sepsis-inducing microbes but their overuse is contributing to the threat of drug-resistant microbes and they don’t neutralize the toxins that some pathogens leave behind. Commercial dialysis equipment can remove toxins from the blood but is not built for routine use in theater.
DARPA’s Dialysis-Like Therapeutics (DLT) program seeks to develop integrated, portable and ruggedized technology that would enable widespread deployment of dialysis treatment to fight sepsis. The program recently tested a novel prototype that could greatly advance sepsis treatment and lead to increased survival of future sepsis patients. As reported in Nature Medicine, the DLT program successfully demonstrated one of the first technologies for pathogen removal via blood filtration. With a design inspired by the human spleen, the shoebox-sized prototype removes many of the microbes and toxins that can trigger sepsis.
“Sepsis is a massive problem for both civilian and military healthcare, which is why DARPA set out to develop more effective and portable technologies for sepsis treatment,” said U.S.Army Col. Matt Hepburn, DARPA program manager. “Our ‘artificial spleen’ prototype shows a promising new way to fight sepsis more quickly and thoroughly. The technology is also small and light, and usable either on its own or with commercial dialysis equipment.”
Eventually, Hepburn added, DLT could preclude the need to fully identify a bacterium or test whether it is antibiotic resistant—which can take hours or days—before beginning treatment. “We’re hopeful that this new technology could give doctors new tools to save lives in the future,” he said.
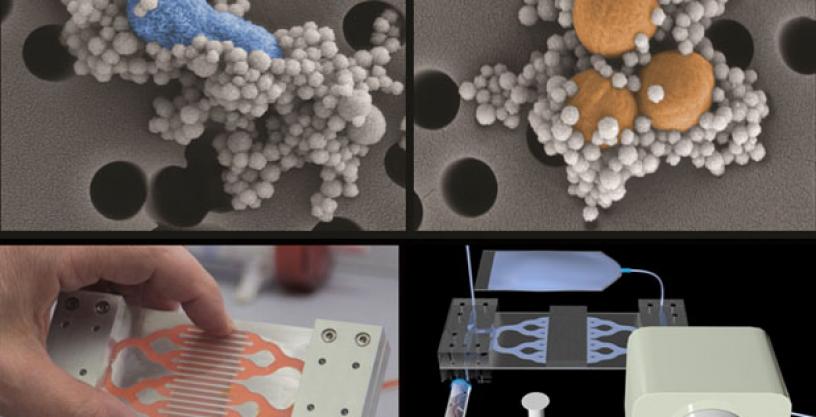
The DLT prototype is designed to work outside the body like a traditional dialysis machine. Blood is funneled from a patient’s veins into the device, where it is cleaned before returning to the body. The approach is similar to that used by the human spleen, which removes pathogens and dead cells from the blood by filtering it through a series of tiny interwoven blood channels.
In the DLT device, a series of microfluidic channels take the place of blood vessels. Two adjacent hollow channels—one containing flowing blood, the other a saline solution—connect to each other by a series of slits. As blood enters the device, it is mixed with tiny magnetic beads 128 nanometers in diameter that are coated with a genetically engineered version of a natural immune system protein called mannose binding lectin (MBL). Sepsis-inducing microbes and toxins stick to the beads, which a magnet pulls through the slits and into the saline solution for collection and removal. The cleaned blood is then re-infused into the patient.
DLT’s breakthrough capabilities capitalize on the structure of MBL, which has a branch-like “head” and a stick-like “tail.” In the body, the head binds to specific sugars on the surfaces of harmful microbes and toxins, and the tail attaches to immune-system proteins that catalyze their destruction. Sometimes, however, the tails attach to other immune system proteins, which activate an autoimmune response that can lead to blood clotting and organ damage. The DLT team used genetic engineering tools to create an altered version of MBL whose tail does not bind to the problematic, auto-immune-triggering proteins.
The DLT team first tested its blood-cleaning system in the laboratory using human blood spiked with pathogens. A single device bound and removed more than 90 percent of key sepsis pathogens. The team next tested the device using rats infected with two pathogens (E. coli and S. aureus) along with bacterial toxins—a microbial cocktail mimicking those seen in the bloodstreams of human sepsis patients. The prototype removed about 90 percent of the bacteria and toxins from the rats’ bloodstreams within five hours.
Going forward, DARPA plans to continue development of component technologies to mitigate sepsis and then combine those component technologies into an integrated system. Subsequent trials would then seek to demonstrate the safety and efficacy of the integrated DLT device and provide crucial evidence to support an eventual application to the U.S. Food and Drug Administration for clinical trials with human volunteers.
# # #
Associated multimedia posted on www.darpa.mil may be reused according to the terms of the DARPA Usage Agreement, available at: http://www.darpa.mil/policy/usage-policy.
Tweet @darpa
